Search results
Search for "reactive radicals" in Full Text gives 6 result(s) in Beilstein Journal of Nanotechnology.
Tin dioxide nanomaterial-based photocatalysts for nitrogen oxide oxidation: a review
Beilstein J. Nanotechnol. 2022, 13, 96–113, doi:10.3762/bjnano.13.7

- SnO2 NPs, and this is the first report on using a SnO2 photocatalyst with NP morphology for the NO degradation. The photocatalytic mechanism of SnO2 NPs is based on electrons and holes to generate reactive radicals. Figure 7 shows that the photocatalytic NO removal efficacy of SnO2 NPs achieved 63.37
Sputtering onto liquids: a critical review
Beilstein J. Nanotechnol. 2022, 13, 10–53, doi:10.3762/bjnano.13.2
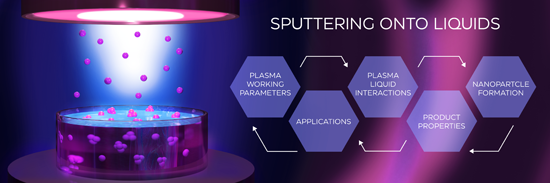
- multiply charged ions (Ar2+, M2+). If the plasma is generated in an argon/reactive gas mixture R+, R2+, R2+, and MRx+ ions (with R being, e.g., O or N) can also be found; (3) chemically reactive radicals such as O or N atoms produced through dissociation reactions during reactive sputtering. O atoms can
Mechanistic insights into plasmonic photocatalysts in utilizing visible light
Beilstein J. Nanotechnol. 2018, 9, 628–648, doi:10.3762/bjnano.9.59

- : localized surface plasmon resonance (LSPR); noble metal; plasmonic photocatalyst; reactive radicals; Schottky junctions; visible light; Review Introduction Photocatalysts have played and will continue to play a pivotal role in environmental and energy applications in order to fulfil the needs of the
- simulation method, it was claimed that the enhanced photo-electrochemical water splitting performance of Pd@BiVO4 was attributed to the hot-electron injection from Pd NPs upon SPR excitation in the vis–NIR region [112]. Identification of reactive radicals It is quite obvious that photocatalysis is supported
Process-specific mechanisms of vertically oriented graphene growth in plasmas
Beilstein J. Nanotechnol. 2017, 8, 1658–1670, doi:10.3762/bjnano.8.166

- dissociates under plasma and forms reactive radicals/ions. Transport mechanism of these plasma species and growth kinetics of carbon nanomaterials in PECVD has been extensively explained by Munoz and co-workers [26]. The density and energy of these plasma species depend on the plasma power, position of the
Photocatalysis applications of some hybrid polymeric composites incorporating TiO2 nanoparticles and their combinations with SiO2/Fe2O3
Beilstein J. Nanotechnol. 2017, 8, 272–286, doi:10.3762/bjnano.8.30

- point, the degradation of organic compounds into CO2 and H2O begins. It is caused by reactive radicals (•OH, •O2−) produced through the interactions between the charge carriers from the surface with the adsorbed molecules (e.g., water and molecular oxygen) [25][54]. For this study, the
Tm-doped TiO2 and Tm2Ti2O7 pyrochlore nanoparticles: enhancing the photocatalytic activity of rutile with a pyrochlore phase
Beilstein J. Nanotechnol. 2015, 6, 605–616, doi:10.3762/bjnano.6.62

- electrons and holes produce a reaction with attached molecules on the material surface and create reactive radicals. The electrons in the conduction band react with O2 to form superoxide anion radicals (O2−•) and the holes in the valence band react with water to form hydroxyl radicals (•OH). The activated






































































































